Citation: if this protocol is useful in your work, please consider citing the paper where it was initially published: Ashhurst T.M., Cox D.A., Smith A.L., King N.J.C. (2019) Analysis of the Murine Bone Marrow Hematopoietic System Using Mass and Flow Cytometry. In: McGuire H., Ashhurst T. (eds) Mass Cytometry. Methods in Molecular Biology, vol 1989.https://doi.org/10.1007/978-1-4939-9454-0_12.
Counting using an automated cell counter, Sysmex XP-100
- Turn machine ON.
- Whilst the machine starts up, transfer 100 µL of sample to a 1-1.5 mL tube.
- Raise the sample tube to the probe so that the probe is submerged in the sample fluid.
- Press the GREEN start button.
- Hold sample tube in place (with the probe submerged) during aspiration until you hear beeping, then remove the tube. The machine will aspirate approx. 60 µL of sample. Leukocyte, or white blood cell (WBC) counts are given as nx109 cells/L (= nx106 cells/mL).
- Calculate cells per sample :
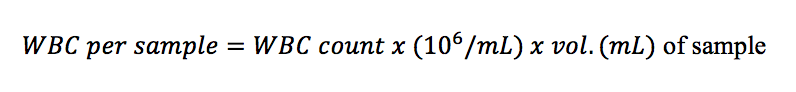
Note: Sysmex XP-100 for counting mouse cells
The Sysmex XP-100 histogram analysis was configured for human blood cells, which are larger than the equivalent mouse cells. When we compared mouse leukocyte counts on the Sysmex XP-100 and haemocytometer, they were reasonably comparable; although the XP-100 was not able to distinguish lymphocytes, monocytes, and granulocytes from one another. However, erythrocyte and thrombocyte counts were inaccurate, and under-reported the true numbers; as is typical of many automated cell counters designed for human blood counting. The default leukocyte concentrations are reported as 109 cells/L, which equates to 106 cells/mL.